 |
Hemant P.Joshi Chief scientist,General manager of IVIVR Ph.D. in Rajiv Gandhi University of Health Science ● He was working as advanced scientist in several famous companies, such as Dr. Reddy‘s Laboratories and Sun pharmaceuticals and has almost 20-year-experience in clinical pharmacy and preparation R&D. ● He was involved in dozens of FDA, EMA registered ANDA, 505b(2) research and development work. ● He was involved in the pharmaceutical R&D and clinical program design of a number of 505b(2) innovative drugs and generic drugs, understanding of pharmacy and clinical practice. |
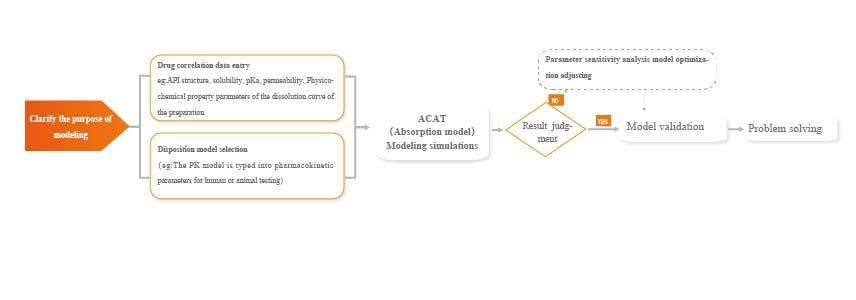
Platform Introduction:
The platform is based on the company's chief scientist, Dr. Hemant P. Joshi, and combines the dissolution characteristics of the drug with the in vivo mechanism of action, modeling through a variety of software to predict in vivo behavior through in vitro data and provide support for pharmaceutical research and clinical research.
1、 Empowering innovative medicines
① PK curve and efficacy prediction② Accelerate clinical research(innovative drug data bridging, clinical trial exemptions)
③ Guiding clinical trial design (initial dose selection, dose selection for specific populations, dosing interval, food effects, drug-drug interactions)
2、Assisting generics drugs
① Virtual equivalence tests to assess the difference in quality between the test formulation and the reference formulation and clarify pharmaceutical goals
② Bioequivalence exemption data support
③ Interpretation of BE test results and optimization guidance.


 Hot line:010-83057670
Hot line:010-83057670
 EN
EN
 010-83057670
010-83057670 Address:
Address: Marketing Department:
Marketing Department: Leadingpharm
Leadingpharm Pharm News
Pharm News 010-61006450
010-61006450
